Mitsui Chemical Inc. of Japan has decided to begin construction of a pilot plant for continued development of producing methanol from industrial CO2 effluent and photocatalyst produced hydrogen. Due to build starting in October of 2008 with completion next February the plant is expected to go into use in March of 2010, the plant’s annual yield would work out to be the U.S. equivalent of over 33,000 gallons.
The construction will be at the company’s Osaka facilities where some 150 to 160 tons of CO2 can be obtained. The new plant is based on the cooperative work of Mitsui and the Research Institute of Innovative Technology for the Earth in Kyoto Japan. Mitsui has a nearly ten-year investment in the effort during the 1990s from which it is bringing a single unit photoelectrocatalyst hydrogen production technology. This process uses a highly efficient thin film, anatase titania photocatalyst that has a photon to current quantum efficiency of 60%. The yield of 220,000 pounds of methanol would need approximately 22% hydrogen by weight, some 48,400 pounds. Where the solar array will be installed isn’t discussed.
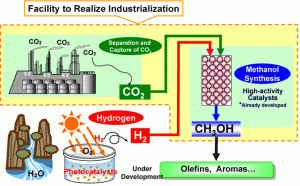
Mitsui Methanol Process Illustration
The CO2 side is from the Mitsui complex’s own process waste stream. The company makes the case that the cause of the research and investment is in mediation of CO2 emissions. It is a fully credible claim, yet the rise in petrochemical prices has to have a role in making the pilot investment worthwhile. The CO2 process is also based in results form the Research Institute’s “Chemical CO2 Immobilization Project.” Here Mitsui participated and took the research further with proprietary ultra high activity catalysts. This base research was in zinc oxide and copper catalysts that are upgraded to yield 44% efficiency for methane and 24% for ethylene from 82% of the carbon dioxide feed. This published rate does not deactivate the catalysts when operating in a pulsed bias.
The dollar investment is published at US$13.7 million or only $62.28 per annual gallon of capital cost. Without an operating expense it isn’t known how viable this venture might be, but the graph above notes that the output will be directed to valued added processes to yield things like olefins and aromatics.
The vague area is in the costs to sequester the CO2 and the use of the excess oxygen. It seems sensible that the unused 18% CO2 from the process would loop back in. The oxygen has value in O2 form and it’s a sure thing it won’t be vented as ozone.
Pilot plants are the first level scale up from the lab bench stage to check the real world practice and costs before trying an industrial sized plant. It’s quite interesting to see a number like less than $63.00 of capital costs even before the running expenses are factored in. What part of the capital costs would be the instrumentation that would not go forward to industrial sized builds isn’t known nor the other special one off costs that come from a first build.
What is illustrated is that in Japan at least, the sense that CO2 can be recycled back into the petrochemical stream is a viable idea that merits engineering and construction investments. It’s also noteworthy that they understand the importance of providing a source of hydrogen to maximize the product yield. The 82% CO2 used number is just outstanding. This is a much better concept than the US effort to somehow sequester and bury CO2. A few months ago the U.S. dropped a coal plant sequestration effort as it was simply too expensive. It is much smarter to make use of the value in the waste CO2 than try to make another kind of “landfill” for CO2. CO2 actually has value, as any green plant can tell you, and when processed up to a value added product it can stop being a topic of such contention.

No comments:
Post a Comment